Disclaimer on Maximum Dose Recommendations for Local Anesthetics
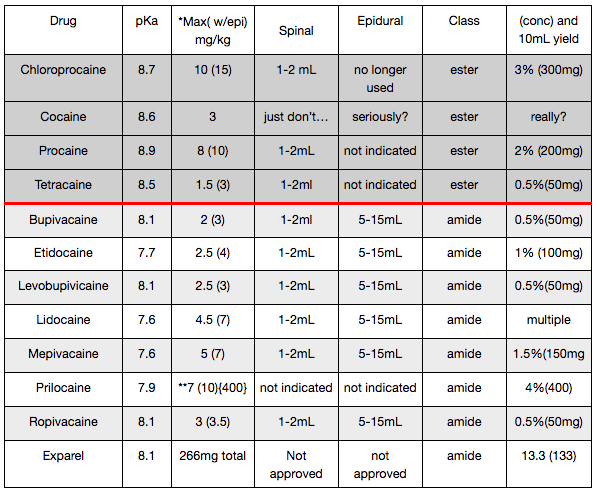
The maximum dose regimens presented herein are based on a synthesis of multiple reputable sources, which themselves differ in their conclusions. It is important to understand that these values are not absolute. Instead, they serve as general guidelines and should be adjusted according to individual patient variables—including desired onset speed, necessity for motor blockade, anticipated duration of effect, comorbid conditions, nutritional and protein status, and other clinical considerations. These figures are intended for reference only and do not replace clinical judgment.
Final dosing decisions remain the responsibility of the individual provider. Twin Oaks Anesthesia assumes no responsibility or liability for adverse outcomes resulting from anesthetic selection or dosing.
Note: The epidural dose ranges referenced are based on the assumption of a lumbar epidural requiring an initial bolus. Given the complexity of epidural anesthetic management, it is inappropriate to rely solely on these values without accounting for patient-specific and procedural factors.
Prilocaine Advisory: The maximum recommended dose of prilocaine for adults is approximately 400 mg, primarily due to the risk of methemoglobinemia. Please refer to the Local Anesthetics chapter for detailed guidance on the recognition and management of this condition.
Fundamentals of Local Anesthetics in Regional Anesthesia
Understanding the pharmacologic properties of local anesthetics is foundational to the safe and effective practice of regional anesthesia. Each agent possesses unique characteristics that can influence block onset, duration, and depth. Central to their mechanism is the blockade of voltage-gated sodium channels, which disrupts nerve conduction by altering membrane permeability primarily to sodium, and to a lesser extent, potassium ions. Although the precise molecular interactions are not fully understood, the sodium channel remains the primary target of local anesthetic action.
Key Pharmacologic Principles
1. Hydrophobicity (Lipid Solubility) Local anesthetics are weak bases formulated with hydrochloride salts to enhance water solubility. However, only the non-ionized, lipid-soluble portion can traverse the lipophilic neuronal membrane. Once inside the nerve cell, these molecules become ionized and bind to the sodium channel, stabilizing it in an inactive state and preventing further nerve impulse propagation. Notably, distal responses (e.g., motor responses to nerve stimulation) can still occur beyond the block site, so further needle advancement must be approached cautiously to avoid intraneural injection.
2. Protein Binding Local anesthetics bind primarily to alpha-1-acid glycoprotein (AAG) and, to a lesser degree, albumin. High-affinity, low-capacity binding to AAG occurs first, followed by albumin binding. Binding efficiency is pH-dependent: acidosis reduces protein binding and increases free (active) drug concentrations, elevating the risk for systemic toxicity.
3. pKa and Onset The pKa of a local anesthetic reflects the pH at which it exists in equal ionized and non-ionized forms. Most local anesthetics have a pKa between 7 and 9. The non-ionized form determines membrane permeability and, therefore, block onset. Increasing the pH of the solution (e.g., with bicarbonate) enhances the proportion of non-ionized drug, accelerating onset. Conversely, acidic environments (e.g., infected tissue) favor ionization, reducing efficacy.
Local Anesthetic Toxicity (LAST)
Systemic toxicity may result from rapid absorption or accidental intravascular injection. Symptoms range from early signs such as circumoral numbness, tinnitus, and metallic taste, to severe manifestations including seizures, coma, cardiovascular collapse, and death. Resuscitation may be difficult due to strong anesthetic binding to cardiac sodium channels.
Patients with hypoalbuminemia or those undergoing blocks involving highly vascularized tissues are at increased risk. For example, adductor canal blocks expose anesthetics to three vascular surfaces, enhancing absorption, while the IPACK block involves bony surfaces with limited uptake.
Management of LAST Lipid emulsion therapy (e.g., 1–1.5 mL/kg of 20% intralipid) has been shown to be effective in treating severe LAST. Proposed mechanisms include sequestration of lipophilic agents and restoration of fatty acid metabolism in cardiac tissue.
Hypersensitivity and Allergic Reactions
Although rare, hypersensitivity to local anesthetics can be serious. Reactions are classified as:
Type I (IgE-mediated): Immediate onset within 30 minutes; symptoms include urticaria, bronchospasm, hypotension, and anaphylactic shock. Treatment involves antihistamines, corticosteroids, epinephrine, and airway support.
Type IV (T-cell-mediated): Delayed onset (up to 48 hours); symptoms include localized erythema and edema.
Most true allergic reactions occur with ester-type anesthetics or preservatives (e.g., parabens, sulfites, latex). Nickel exposure from needles and other sources may also be implicated.
Other confounding causes of adverse reactions include toxic overdose, anxiety-induced responses, vasovagal syncope, and reactions to additives.
Methemoglobinemia
This condition involves oxidation of hemoglobin iron from the ferrous (Fe2+) to the ferric (Fe3+) state, impairing oxygen release. It can be triggered by agents such as prilocaine or benzocaine. Clinical symptoms include cyanosis, hypoxia, and altered mental status.
Treatment: IV methylene blue (1–2 mg/kg) rapidly reduces methemoglobin back to functional hemoglobin.
Clinical Considerations
Maintain awareness of patient-specific risk factors (e.g., protein status, comorbidities).
Thoroughly review patient history and consider allergy consultation when warranted.
Document all adverse events and responses.
Note: Prilocaine dosing should not exceed 400–500 mg in adults due to its strong association with methemoglobinemia.
A comprehensive understanding of these pharmacologic principles enhances the safe application of regional anesthesia and minimizes complications.
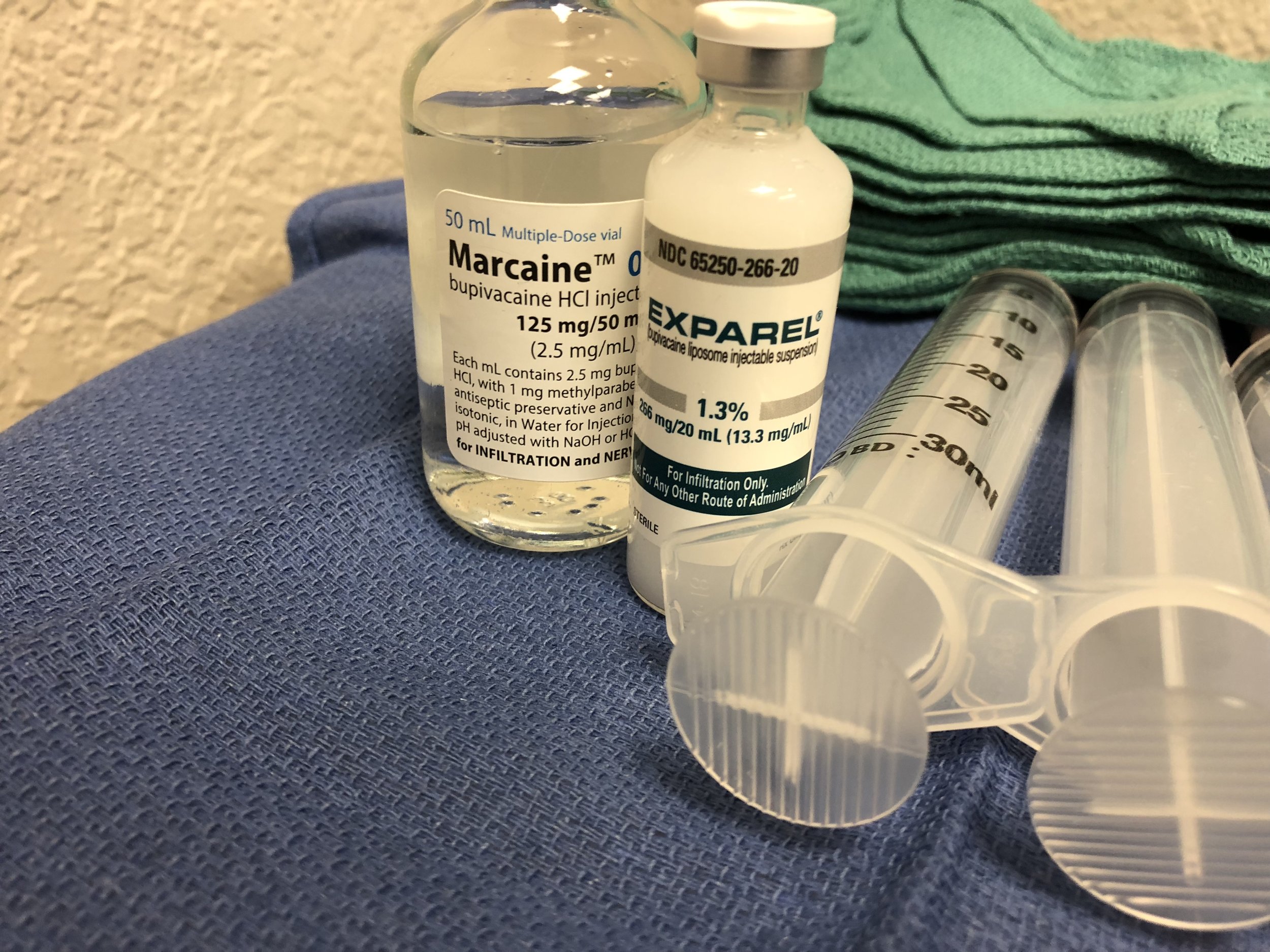
Exparel: Liposomal Bupivacaine Delivery System
Exparel represents a novel advancement in local anesthetic delivery. It is a liposomal formulation of bupivacaine (commonly referred to as marcaine), designed to provide extended analgesia through sustained release. The active drug is encapsulated within a multivesicular liposomal structure known as DepoFoam, which enables gradual dispersion of the anesthetic into surrounding tissues following injection.
This slow-release delivery platform is not entirely new. Earlier iterations include DepoCyt, a liposomally encapsulated formulation of cytarabine used as an antineoplastic agent, and DepoDur, a liposomal epidural morphine product. In Exparel, this same liposomal technology is applied to bupivacaine, offering the potential for analgesia lasting up to 96 hours, as supported by pharmacokinetic studies and early clinical data.
Pharmacodynamic analysis reveals that Exparel results in a significantly reduced peak plasma concentration when compared to traditional formulations of bupivacaine, which may contribute to its improved safety profile.
Despite promising results, Exparel is not currently FDA-approved for use in peripheral nerve blocks. It is formally indicated only for surgical site infiltration. Nevertheless, off-label use has been reported in several interfascial plane blocks, including TAP, PECs II, erector spinae, quadratus lumborum, cervical plexus, and trigger point injections, with anecdotal and case report-level evidence suggesting efficacy and safety.
It is essential to emphasize that Exparel must not be mixed with any other local anesthetic except bupivacaine. This restriction is due to the potential for competitive displacement from the liposomal structure, which could result in premature release and altered pharmacokinetics.
Another important consideration is concentration: while standard bupivacaine is commonly available in concentrations of 0.25% to 0.5%, Exparel is supplied at 13.3 mg/mL. This higher concentration, paired with its unique delivery system, warrants careful attention to dosing protocols.
(Refer to the accompanying image for a microscopic view of a disintegrating Exparel liposomal particle.)