Erector Spinae Plane (ESP) Block
With the increasing prevalence of thoracic, abdominal, and reconstructive procedures, the demand for safe and effective analgesia targeting the trunk has grown substantially. Historically, thoracic and lumbar epidurals were considered the gold standard for postoperative pain control in these regions. However, their inherent risks, contraindications (such as coagulopathy), and technical demands have prompted the exploration of alternative regional techniques. Among these alternatives are the PECS blocks, thoracic paravertebral blocks, and targeted transverse abdominis plane (TAP) blocks. More recently, the erector spinae plane (ESP) block has emerged as a promising and relatively straightforward technique for providing analgesia to the thoracic and abdominal walls.
The ESP block leverages the anatomical features of the paraspinal musculature, specifically the fascial plane deep to the erector spinae muscle and superficial to the transverse processes. While the bony landmarks of this region are well characterized, the fascial pathways through which sensory nerves traverse—particularly the cutaneous branches and interconnecting channels—have only recently been better understood.
Compared to its more technically demanding counterparts—such as the paravertebral block, quadratus lumborum (QL) blocks, and the serratus anterior plane block—the ESP block offers a favorable safety profile, minimal risk of neuraxial spread, and a simplified approach. Despite variations in technique and target location among these blocks, they appear to provide similar dermatomal coverage and analgesic efficacy. This observation has led to evolving theories regarding the mechanism of action, suggesting that extensive longitudinal spread within the paraspinal fascial plane can allow access to both dorsal and ventral rami, including the rami communicantes that carry sympathetic fibers.
Emerging evidence also challenges earlier assumptions about local anesthetic distribution. Rather than diffusing laterally, studies have shown that injectate remains confined within the paraspinal gutter, yet can still provide both somatic and visceral analgesia. This finding has significant implications, particularly for patients for whom neuraxial techniques are contraindicated, such as those on anticoagulant therapy.
The ability of ESP blocks to target both cutaneous and visceral components further aligns them with the QL block, making them unique in their class. This has led to speculation that previously underappreciated connective tissue pathways between layers of the posterior thoracic musculature may enable anesthetic spread to multiple nerve branches as they course through the region.
Current Indications and Clinical Utility
While indications for the ESP block continue to evolve, existing literature supports its efficacy in providing postoperative analgesia for procedures involving the chest, breast, and abdominal wall. Its ability to provide both somatic and visceral pain relief adds to its versatility and positions the ESP block as a valuable tool in modern regional anesthesia, particularly for patients at higher risk from traditional neuraxial techniques.
Anatomy
The term erector spinae refers not to a single anatomical structure, but rather to a functional muscle group responsible for maintaining spinal extension and posture. This group includes the iliocostalis lumborum and longissimus thoracismuscles, among others. The erector spinae plane (ESP) block targets the fascial plane deep to these muscles, specifically focusing on the anatomic relationship between the three posterior muscle layers (latissimus dorsi, rhomboid major, and erector spinae group) and the dorsal rami of spinal nerves.
These dorsal rami give rise to cutaneous branches that innervate the posterior thoracic region as well as portions of the anterolateral chest wall. Historically, it was believed that accessing these nerves required penetration into the paravertebral space, as they course through the neural foramen and into the paravertebral gutter. However, more recent anatomical and clinical insights suggest the existence of interfascial channels between the layers of the paraspinal musculature, which allow local anesthetic to spread both cephalad and caudad over multiple spinal levels without entering the paravertebral space directly.
These posterior musculature layers appear to form a longitudinal conduit extending from the cervical to the lumbar spine, facilitating effective anesthetic spread along the dorsal nerve pathways. Notably, the pectoral musculature(pectoralis major and minor) and sternal region are not innervated via this pathway and remain outside the coverage area of the ESP block.
Technique
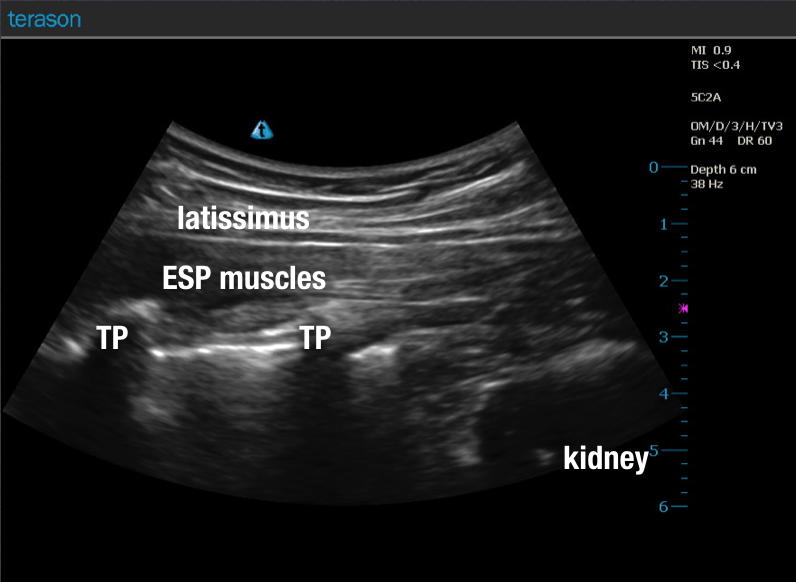
Although slight variations exist, the ESP block is consistently performed under ultrasound guidance to ensure safe and accurate needle placement. A high-frequency linear probe is typically preferred for patients with normal body habitus, while a low-frequency curved array probe may be more suitable for obese patients or deeper target structures.
The objective is to deposit local anesthetic deep to the erector spinae muscle and superficial to the transverse processat the desired vertebral level, thereby enabling longitudinal spread within the fascial plane. The procedure generally begins with an axial (transverse) ultrasound scan to visualize key osseous landmarks, including the spinous process and the transverse process. Once the transverse process is clearly identified, the probe is rotated longitudinally (parasagittal orientation) to facilitate an in-plane needle approach and allow precise deposition of anesthetic into the target plane.
See Gallery 35.1 for representative ultrasound images demonstrating correct probe placement, anatomical landmarks, and needle trajectory.
Once the transverse process at the target level has been clearly identified, the ultrasound probe should be repositioned into a longitudinal parasagittal orientation, ensuring visualization of the tip of the transverse process and the overlying paraspinal musculature, typically seen in two to three distinct layers. This view facilitates accurate needle placement and optimal distribution of local anesthetic. Notably, early clinical reports have demonstrated that a single injection at the T5 level may result in cephalocaudal spread from T1 through T8, providing substantial thoracic coverage.
Following sterile preparation of the skin and transducer in accordance with institutional protocol, the anticipated needle entry site should be anesthetized using a fast-acting local anesthetic such as 1% lidocaine. This not only reduces patient discomfort but may also improve ultrasound image quality by enhancing acoustic contrast.
A block needle is then introduced in-plane under continuous ultrasound guidance, advancing toward the lateral aspect of the transverse process. Both cephalad-to-caudad and caudad-to-cephalad needle trajectories have been described successfully in the literature, indicating that the specific direction of approach may be of minimal clinical importance.
After confirming negative aspiration, local anesthetic is deposited within the erector spinae plane, between the deep surface of the erector spinae muscle and the dorsal surface of the transverse process. It is critical to ensure proper deposition at this fascial interface, as intramuscular injection is not desired. The displacement of the erector spinae muscle anteriorly, away from the transverse process, along with the formation of an anechoic stripe connecting adjacent transverse processes, are reliable indicators of correct needle placement.
A volume of 20 mL of local anesthetic per side is commonly cited in the literature, though this may be adjusted based on the patient’s body habitus, the extent of desired dermatomal spread, and the presence of adjunct techniques (e.g., tumescent anesthesia). While some studies note that a single-injection ESP block may have limited duration, the addition of adjuncts such as dexamethasone has shown promise in prolonging analgesic effects.
When used in conjunction with other regional or local techniques, such as tumescent infiltration, the inclusion of epinephrine may further enhance safety by delaying systemic uptake and reducing plasma concentrations of long-acting local anesthetics like bupivacaine or ropivacaine.
Some practitioners have reported success with initial single-shot injections followed by the placement of indwelling catheters for extended postoperative analgesia. Given that rapid onset is typically not the primary goal when used alongside general anesthesia, a lower concentration, long-acting local anesthetic (e.g., ropivacaine 0.2–0.375%) in volumes of 20 mL is often sufficient for adults. As motor blockade is not an intended outcome, high concentrations are unnecessary and may be avoided to optimize safety margins and accommodate larger volumes if needed elsewhere.
The procedural notes provided are intended as foundational tools to support and streamline clinical documentation. Users are encouraged to modify the language in accordance with their institutional policies and individual practice needs. These notes are not intended for exclusive or unaltered use. Twin Oaks Anesthesia assumes no liability for procedural performance or modifications made to this content.